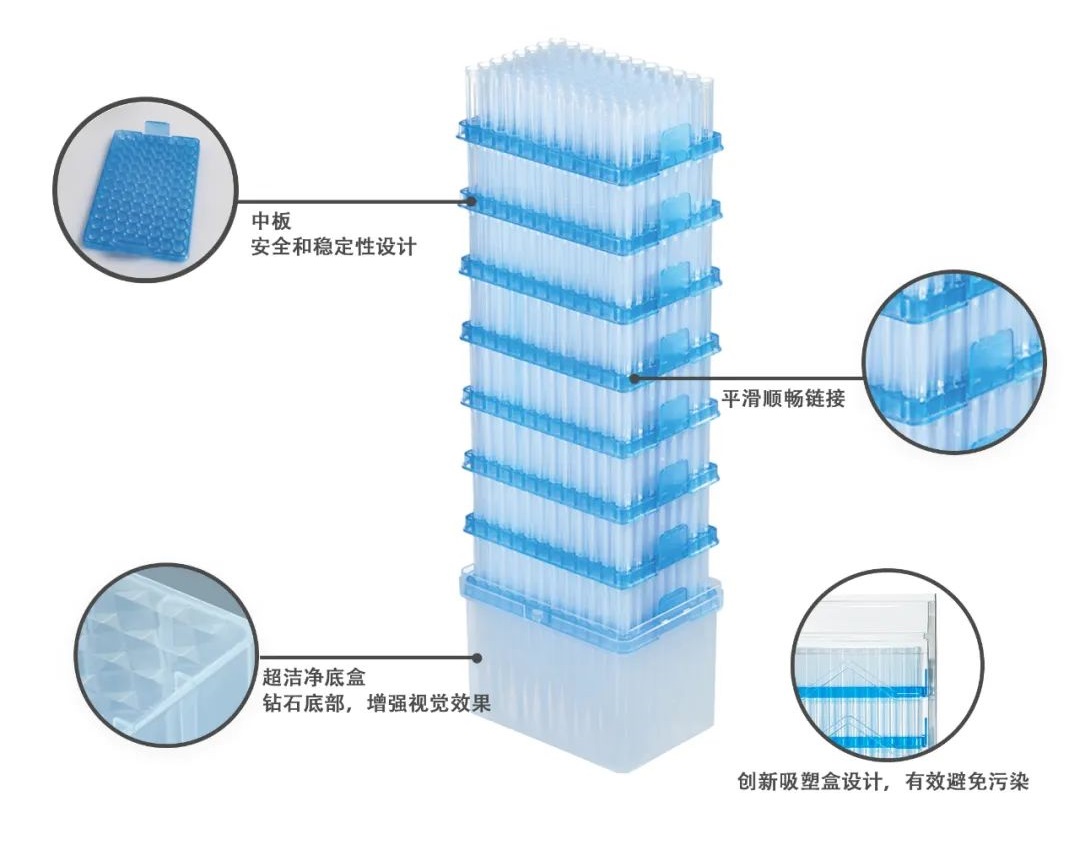
- Universal Pipette Tips
- Low Retention Pipette Tips
- LTS Pipette Tips
- 5ml/10ml Tips
- Robitic Pipette Tips
- Gel Loading Tip
- Serological Pipette
- Cell Culture Flask
- Cell Culture Plate
- Cell Culture Dish
- Petri Dish
- Square Media Bottle
- Erlenmeyer Flask
- Cell Factory System
- Cell Factory accessories
- 96-Well Non-Skirted PCR Plates
- 96-Well Half Skirt, Fit ABI PCR Plates
- 96-Well Half Skirt, Fit Biorad/Roche PCR Plates
- 96-Well Sub Semi-skirt/High-skirt, Fit ABI PCR Plates
- 96-Well Full-skirt PCR Plates
- 40ul 384-Well PCR Plate
- 8-Strip PCR Tubes
- PCR Tubes with Attached Flat Caps
- PCR Sealing Film
- 96 Well Plate
- 96 Well Microplate Plate Deep-Well Tip Combs
- Deep Well I-plate
- 8 Strip Tip Deep-Well Tip Deep-Well Tip Combs
- 10ul Standard 32mm Universal Pipette Tips
- 10ul Extra Long 46mm Universal Pipette Tips
- 10ul Extra Long, Thin and Sharp 46mm Universal Pipette Tips
- 200ul Standard Universal Pipette Tips
- 200ul Standard Wider Universal Pipette Tips
- 200ul Extra Long or 300ul Universal Pipette Tips
- 200 Super Long Universal Pipette Tips
- 1000ul Standard Universal Pipette Tips
- 1000ul Extra Long or 1250ul Universal Pipette Tips
- 1000 Medium Long Universal Pipette Tips
- 10ul Standard 32mm Low Retention Pipette Tips
- 10ul Extra Long 46mm Low Retention Pipette Tips
- 10ul Extra Long, Thin and Sharp 46mm Low Retention Pipette Tips
- 200ul Standard Low Retention Pipette Tips
- 200ul Standard Wider Low Retention Pipette Tips
- 200ul Ultra Long or 300ul Low Retention Pipette Tips
- 200 Super Long Low Retention Pipette Tips
- 1000ul Standard Low Retention Pipette Tips
- 1000ul Extra Long or 1250ul Low Retention Pipette Tips
- 1000 Medium Long Low Retention Adjustable Spacer Multichannel Pipette Tips
- SPTS01 1ml Serological Pipette
- SPTB01 Serological Pipette 1 ML
- SPTS02 2ml Serological Pipette
- SPTB02 Serological Pipetman
- SPTS05 5ml Serological Pipette
- SPTB05 Serological Pipette 5ml
- SPTS10 10ml Serological Pipette
- SPTS10A Plastic Pipette 10ml
- SPTB10A 25ml Serological Pipette
- SPTB10 25ml Serological Pipette
- SPTS25 50ml Serological Pipette
- SPTB25 Serological Pipette 25ml
- SPTS50 50ml Serological Pipette
- SPTB50 Plastic Serological Pipettes
- CL-F025P T25 Cell Culture Flask
- CL-F075P T75 Cell Culture Flask
- CL-F175P T175 Cell Culture Flask
- CL-F225P T225 Cell Culture Flask
- CL-F025P Cell Culture Flask
- CL-FT025P Cell Culture Flask
- CL-F025V Cell Culture Flask
- CL-FT025V Cell Culture Flask
- CL-F075P Cell Culture Flask
- CL-FT075P Cell Culture Flask
- CL-F075V Cell Culture Flask
- CL-FT075V Cell Culture Flask
- CL-F175P Cell Culture Flask
- CL-FT175P Cell Culture Flask
- CL-F175V Cell Culture Flask
- CL-FT175V Cell Culture Flask
- CL-P006 6 Well Cell Culture Plate
- CL-P012 12 Well Cell Culture Plate
- CL-P024 24 Well Cell Culture Plate
- CL-P048 48 Well Cell Culture Plate
- CL-P096 96 Deep Well Plate
- CL-P006 Cell Culture Plate
- CL-PT006 Cell Culture Plate
- CL-P012 Cell Culture Plate
- CL-PT012 Cell Culture Plate
- CL-P024 Cell Culture Plate
- CL-PT024 Cell Culture Plate
- CL-P048 Cell Culture Plate
- CL-PT048 Cell Culture Plate
- CL-P096 Cell Culture Plate
- CL-PT096 Cell Culture Plate
- CL-D035 35 Mm Cell Culture Dish
- CL-D060 6cm Cell Culture Dish
- CL-D100 10cm Cell Culture Dish
- CL-D035 Cell Culture Dish
- CL-DT035 Cell Culture Dish
- CL-D060 Cell Culture Dish
- CL-DT060 Cell Culture Dish
- CL-D100 Cell Culture Dish
- CL-DT100 Cell Culture Dish
- CL-D150 Cell Culture Dish
- CL-DT150 Cell Culture Dish
- SMB125T Square Media Bottle
- SMB250T Square Media Bottle
- SMB500T Square Media Bottle
- SMB1000T Square Media Bottle
- SMB125G Square Media Bottle
- SMB250G Square Media Bottle
- SMB500G Square Media Bottle
- SMB1000G Square Media Bottle
- EFC-125P Erlenmeyer Flask
- EFC-250P Erlenmeyer Flask
- EFC-500P Erlenmeyer Flask
- EFC-1000P Erlenmeyer Flask
- EFC-125V Erlenmeyer Flask
- EFC-250V Erlenmeyer Flask
- EFC-500V Erlenmeyer Flask
- EFC-1000V Erlenmeyer Flask
- EFG-125P Erlenmeyer Flask
- EFG-250P Erlenmeyer Flask
- EFG-500P Erlenmeyer Flask
- EFG-1000P Erlenmeyer Flask
- EFG-125V Erlenmeyer Flask
- EFG-250V Erlenmeyer Flask
- EFG-500V Erlenmeyer Flask
- EFG-1000V Erlenmeyer Flask
- EFBC-125P Erlenmeyer Flask
- EFBC-250P Erlenmeyer Flask
- EFBC-500P Erlenmeyer Flask
- EFBC-1000P Erlenmeyer Flask
- EFBC-125V Erlenmeyer Flask
- EFBC-250V Erlenmeyer Flask
- EFBC-500V Erlenmeyer Flask
- EFBC-1000V Erlenmeyer Flask
- PCRP-10NS 0.1ml Tube
- Unskirted PCRP-10NS-W Plate
- PCRP-20NS 0.2ml 96 Well Plate
- White PCRP-20NS-W Plates
- STSS-509CN 0.5ml Screw Cap Tubes
- STSS-509CR Screw Cap With O Ring
- STSS-509CB Self Standing Tube
- STSS-509CP Clear Tube With Cap
- STSS-509CY Clear Plastic Storage Tubes With Caps
- STSS-509CG Clear Plastic Tube With Lid
- STSS-509AM Acrylic Tube End Caps
- STSS-510CN 1.5ml Conical Tube
- STSS-510CR 1.5ml Cryotubes
- STSS-510CB 1.5ml Screw Cap Tubes
- STSS-510CP 1.5ml Polypropylene Tube
- STSS-510CY 1.5ml Microcentrifuge Tubes Sterile
- STSS-510CG 1.5ml Microfuge Tubes
- STSS-510AM 1.5ml Amber Tube
- STSS-511CN Screw Cap Tube
- STSS-511CR Screw Cap Tube
- STSS-511CB Screw Cap Tube
- STSS-511CP 2ml Screw Cap Microcentrifuge Tubes
- STSS-511CY 2ml Screw Cap Tube
- STSS-511CG Screw Cap Tubes 2ml
- STSS-511AM Amber Centrifuge Tubes
- STSS-509CNS 0.5ml Screw Cap Tube
- STSS-510CNS 1.5ml Screw Cap Tube
- STSS-511CNS Screw Cap Tube
- WMPB008 Pharma Pet Bottles
- WMPB015 HDPE Bottles For Pharmaceutical
- WMPB030 Wide Mouth HDPE Containers
- WMPB060 HDPE Wide Mouth Jars
- WMPB125 Pharmaceutical Plastic Bottles
- WMPB250 Plastic Bottle For Medicine Packaging
- WMPB500 Wide Mouth Polypropylene Jars
- WMPB1000 Bio Plastic Bottles
- WMPB008A Bio Pet Bottle
- WMPB015A Plastic Reagent Bottle
- WMPB030A HDPE Reagent Bottle
- WMPB060A Pharmaceutical Bottle
- WMPB125A Pharma Pet Bottle
- WMPB250A Pharma Plastic Bottle
- WMPB500A Plastic Pharmaceutical Bottles
- WMPB1000A Pharma Bottle Packaging
- NMPB008 Plastic Bottle For Pharmaceutical
- NMPB015 Small Plastic Pill Bottles
- NMPB030 Clear Pill Bottles
- NMPB060 Large Empty Pill Bottles
- NMPB125 Homeopathic Medicine Plastic Bottles
- NMPB250 Plastic Medicine Bottle
- NMPB500 Plastic Containers For Medicines
- NMPB1000 Plastic Drug Vials
- NMPB008A HDPE Medicine Bottle
- NMPB015A Pet Medicine Bottles
- NMPB030A Medical Plastic Bottles
- NMPB060A Plastic Liquid Medicine Bottles
- NMPB125A Plastic Tablet Bottles
- NMPB250A Amber Plastic Medicine Bottles
- NMPB500A Brown Plastic Medicine Bottles
- NMPB1000A Empty Plastic Medicine Bottles
What are you looking for?